Is Ch33n a Lewis Acid or Base
Write an equation to show the reaction between ethanol C2H5OH and methyllithium CH3Li. Is N CH3__3 a Lewis acid or a Lewis base.
In this reaction the ferric ion Fe3 is acting as a Lewis Acid and the oxalate anion C2O42- as a Lewis Base.
. In aqueous solution at 25C it reacts with water to produce its conjugate acid the dimethylammonium ion CH32NH2 and the hydroxide ion OH-. The overall salt does not donate protons the CH3NH3 ion does to form H3O when the salt is dissociated in water. Dimethylamine CH32NH is a weak base.
Your starting point here will be to write the balanced chemical equation that describes the ionization of the trimethylammonium cation CH33NH the conjugate acid of trimethylamine CH33N. Is trimethylamine a Bronsted base. But as implied earlier it can also be a Lewis base.
Which compound can act as both Lewis acid and Lewis base. SO2 acts as a Lewis acid as well as Lewis base. In a Lewis base nitrogen donates an electron pair to an empty valence orbital of an aluminum atom.
Picture for diagram A. The nitrogen has been quaternized and is no longer available. Trimethyl boron is a Lewis acid as it is capable of accepting a lone pair.
CH3 3N is a lewis base due to the presence of lone pair on nitrogen atom. Learn more about the definition and examples of. AlCl3 CH33N Al-Cl3NCH33In the above example AlCl3 is Lewis acid because it have unoccupied valence orbital and can receive electron pairs from CH33N which is Lewis base.
Posted January 23 2009 edited Hi hopefully someone can help me with this question. Using MO diagrams showing HOMOLUMO interactions show the reaction between the above Lewis acid and Lewis base. Rank N CH_3_3 and NCb in terms of strength and give an explanation for your answer.
You also need to draw the structure of the final product. 1 Classify each as a Lewis acid or base. In chemistry a Lewis base is an ionic substance with nonbonding electrons giving it the ability to donate a pair of electrons to an acid.
Is CH3 3N a Lewis acid or a Lewis base. Is B CH3 3 a strong Lewis acid. See the answer See the answer done loading.
CO SO3 CO2 CN- CH33N I- Mn2. It is used to show how the electrons are arranged around individual atoms in a molecule. Give a brief explanation for your answer.
The lone pair of electrons present on the nitrogen can be donated to a Lewis acid. CH33NBH3 2 See answers. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule.
In this reaction ethanol is a Lewis acid and CH3Li is a Lewis base. Similarly carbon monoxide can do that too on the carbon just focus on the first step. Is ch3nh3 a weak base.
Its needs to only 1 electron to complete its octet. Methylamine H3CNH2 dimethylamine H3C2NH and trimethylamine H3C3N are all Bronsted bases and Lewis bases by virtue of the lone electron pair on the nitrogen centre. A Lewis base is a species with an available reactive pair of electrons and a Lewis acid is an electron pair acceptor.
CH3NH3Cl is an ionic compound consisting of CH3NH3 and Cl- ions. Cl- is a very weak conjugate base so its basicity is negligible. By definition a Lewis Base is an electron pair donor whereas a Bronsted base is a proton acceptor.
Find an answer to your question Identify the Lewis acid in this balanced equation. In chemistry a Lewis base is an ionic substance with nonbonding electrons giving it the ability to donate a pair of electrons to an acid. CH33NBH3 morgantaylor112 morgantaylor112 03172020 Chemistry Middle School answered Identify the Lewis acid in this balanced equation.
CH3 3N is a lewis base due to the presence of lone pair on nitrogen atom. Methylamine H3CNH2 dimethylamine H3C2NH and trimethylamine H3C3N are all Bronsted bases and Lewis bases by virtue of the lone electron pair on the nitrogen centre. All the 3 H atoms are bonded with the sp3 hybrid electrons of C but only one electron of C is unpaired.
Draw all non-bonding electrons and show electron flow with curved arrows. Electrons are shown as dots or for bonding electrons as a line between the two atoms. It acts as a Lewis base.
This problem has been solved. The simplest reaction is for a Lewis acid to interact with a Lewis base to give a Lewis acidbase complex. By definition a Lewis Base is an electron pair donor whereas a Bronsted base is a proton acceptor.
Picture for diagram A. Like oxygen the sulfur atom in CH32S has two lone pairs. A Lewis Acid is an electron pair acceptor and a Lewis Base is an electron pair donor.
Lewis Acid Lewis Base Lewis AcidBase Complex. Answer 1 of 2. Boron trifluoride is a very strong Lewis acid but trimethylboron BCH33 is a mild Lewis acid.
Thus CH32S donates an electron pair on sulfur to the boron atom of BH3. Is CH3 3N a Lewis acid. Methylamine H_3CNH_2 dimethylamine H_3C_2NH and trimethylamine H_3C_3N are all Bronsted bases.
This compound is called methylamine and it is basic in nature. Learn more about the definition and examples of a Lewis. Why CH3 3N is a strong Lewis base.
What is a Lewis structural formula. What type of electrolyte is CH3NH2. Is ch33n a Lewis acid or base.
The Lewis base is CH32S and the Lewis acid is BH3. AlCl3 CH33N ÃÂ Â Al-Cl3NCH33In the above example AlCl3 is Lewis acid because it have unoccupied valence orbital and can receive electron pairs from CH33N which is Lewis base. Trimethylammonium ion H_3C_3NH is clearly least able to function as a Lewis Base.
Can CH3NH2 act as a Lewis base. Therefore the salt is acidic because of CH3NH3 a Bronsted acid. In modern theoretical language the Lewis acids LUMO its Lowest.
As in the reaction shown in Equation 821 CO2accepts a pair of electrons from the O2ion in CaO to form the carbonate ion. In a Lewis base nitrogen donates an electron pair to an empty valence orbital of an aluminum atom. Is CH3 2NH a Lewis acid or base.
What is the conjugate acid of CH3 3N. Since Base strength is a measure of tendency to donate lone pair Lewis definition of bases Tri methyl amine is stronger base as it can donate its lone pair to other atoms. Since carbon monoxide has a method for accepting electrons it by definition can be a Lewis acid.
Hence according to Lewis concept it is acidic. By definition a Lewis Base is an electron pair donor whereas a Bronsted base is a proton acceptor.
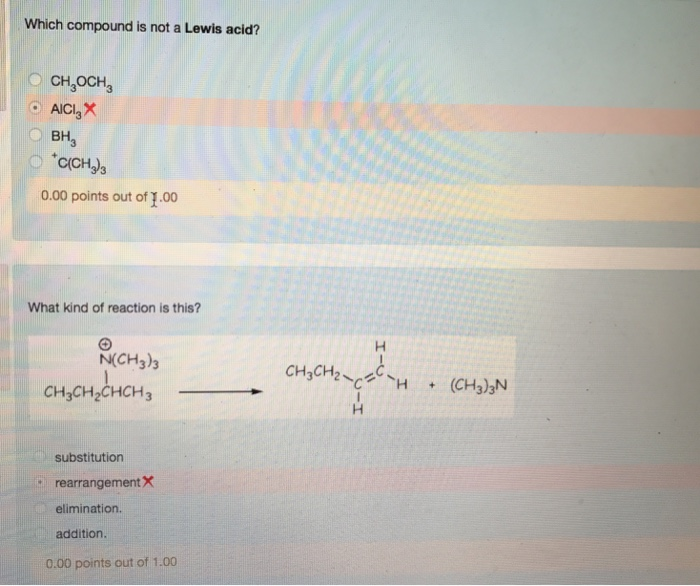
Solved Which Compound Is Not A Lewis Acid Ch Och 3 Obh3 Chegg Com
Is Ch3 3n An Acid Or A Base Quora

Solved Classify Each Species As Either Lewis Acid Or Lewis Chegg Com
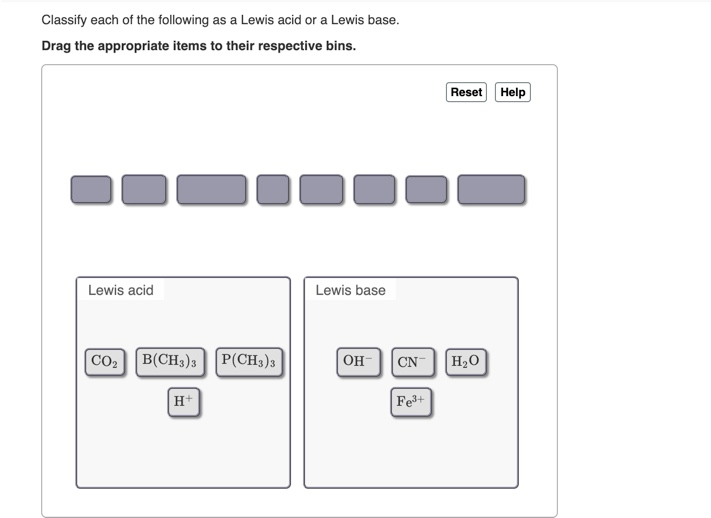
Solved Classify Each Of The Following As A Lewis Acid Or A Chegg Com
Is Ch3 3n An Acid Or A Base Quora

Answered Identify The Lewis Acid And Lewis Base Bartleby

1 8 Chemistry 2810 Answers To Assignment 3 Topic Lewis

14 2 Lewis Acids And Bases General College Chemistry Ii

Solved Using The Lewis Concept Of Acids And Bases Identify Chegg Com

Lewis Acids And Bases Chemistry Classroom Chemistry Education Chemistry Lessons
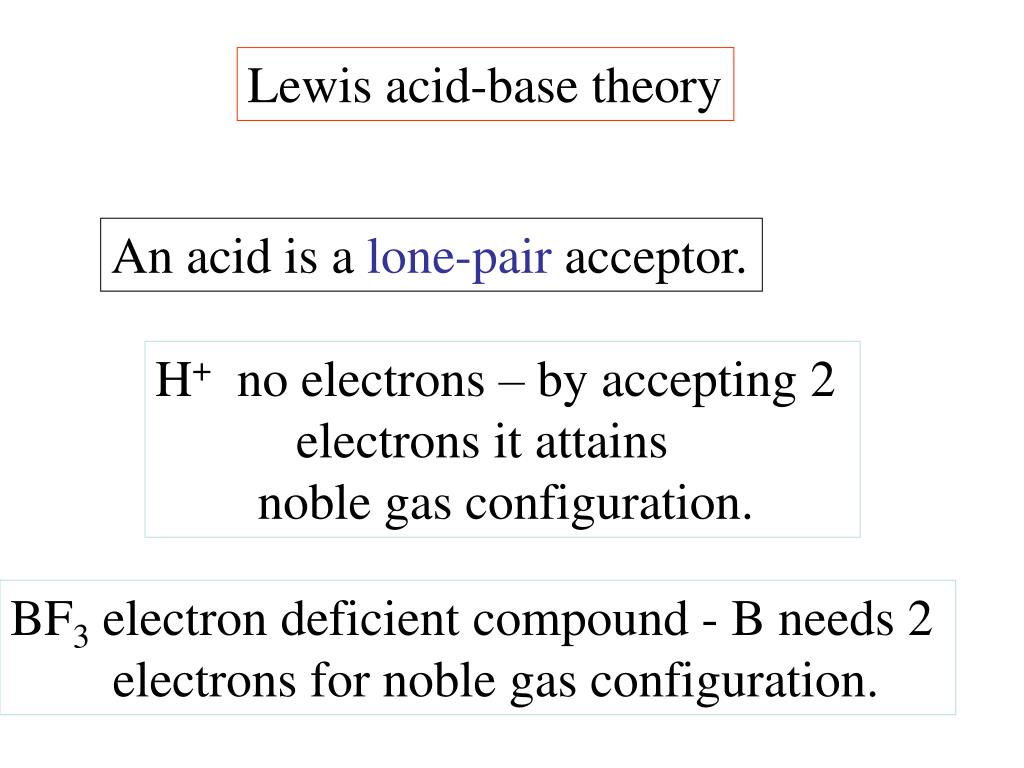
Ppt Lewis Acid Base Theory Powerpoint Presentation Free Download Id 3327585

Classify The Following Species Into Lewis Acids And Lewis Bases And Show How These Act As Lewis Acid Base A Oh B F C H D Bcl3

Classify Each Of The Following As A Lewis Acid Or A Lewis Base Drag The Appropriate Homeworklib
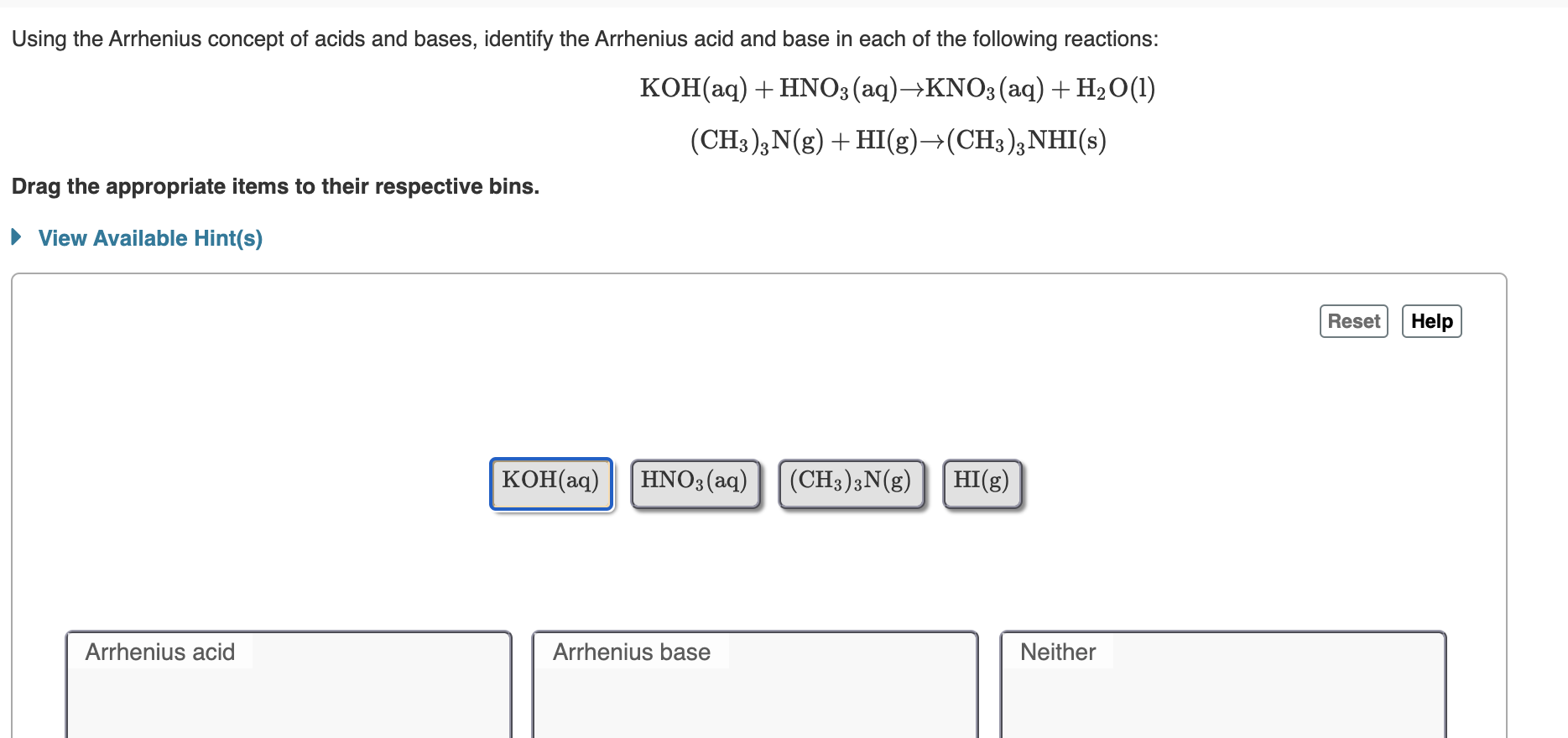
Solved Using The Lewis Concept Of Acids And Bases Chegg Com
Is Ch3 3n An Acid Or A Base Quora
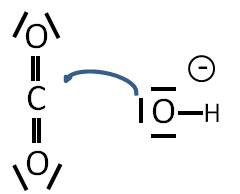
Lewis Acids And Bases Writework
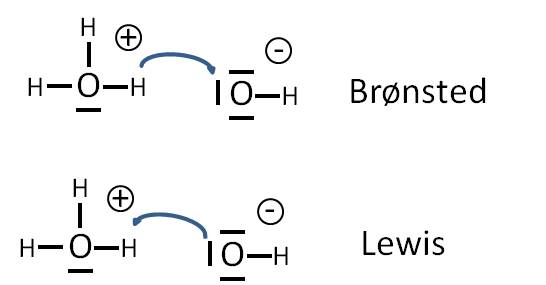
Lewis Acids And Bases Writework
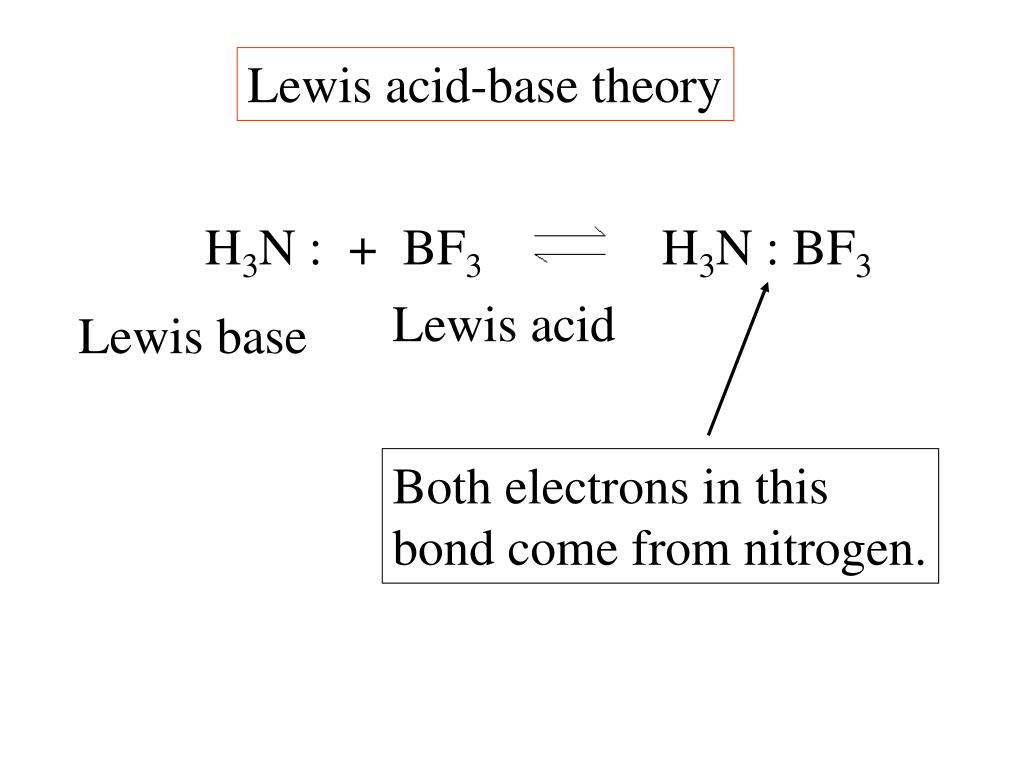
Ppt Lewis Acid Base Theory Powerpoint Presentation Free Download Id 3327585

Comments
Post a Comment